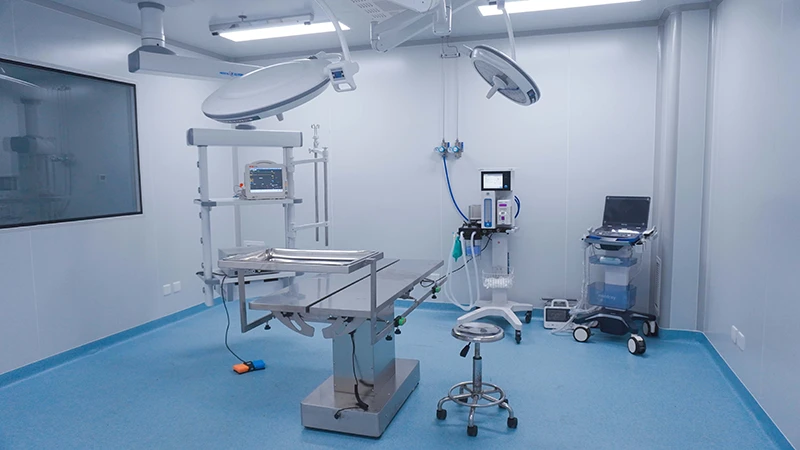
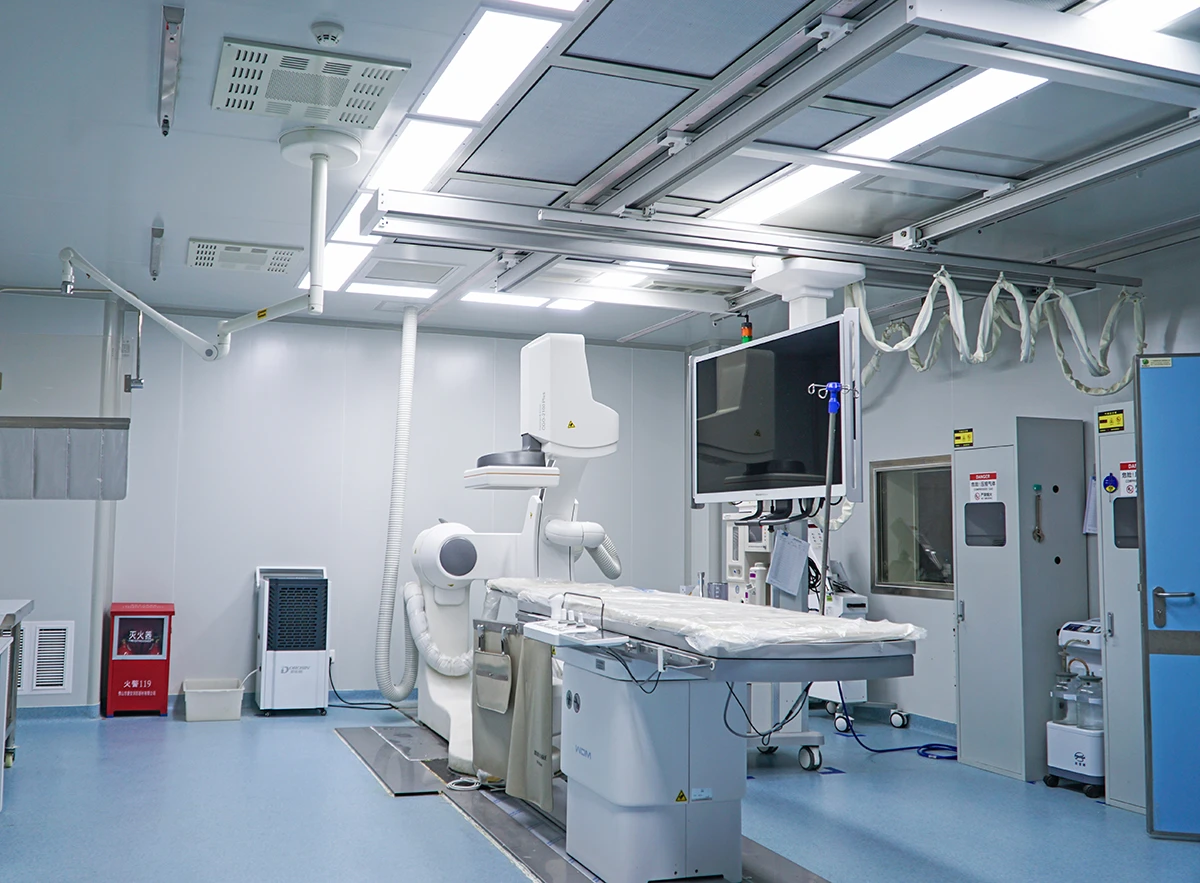
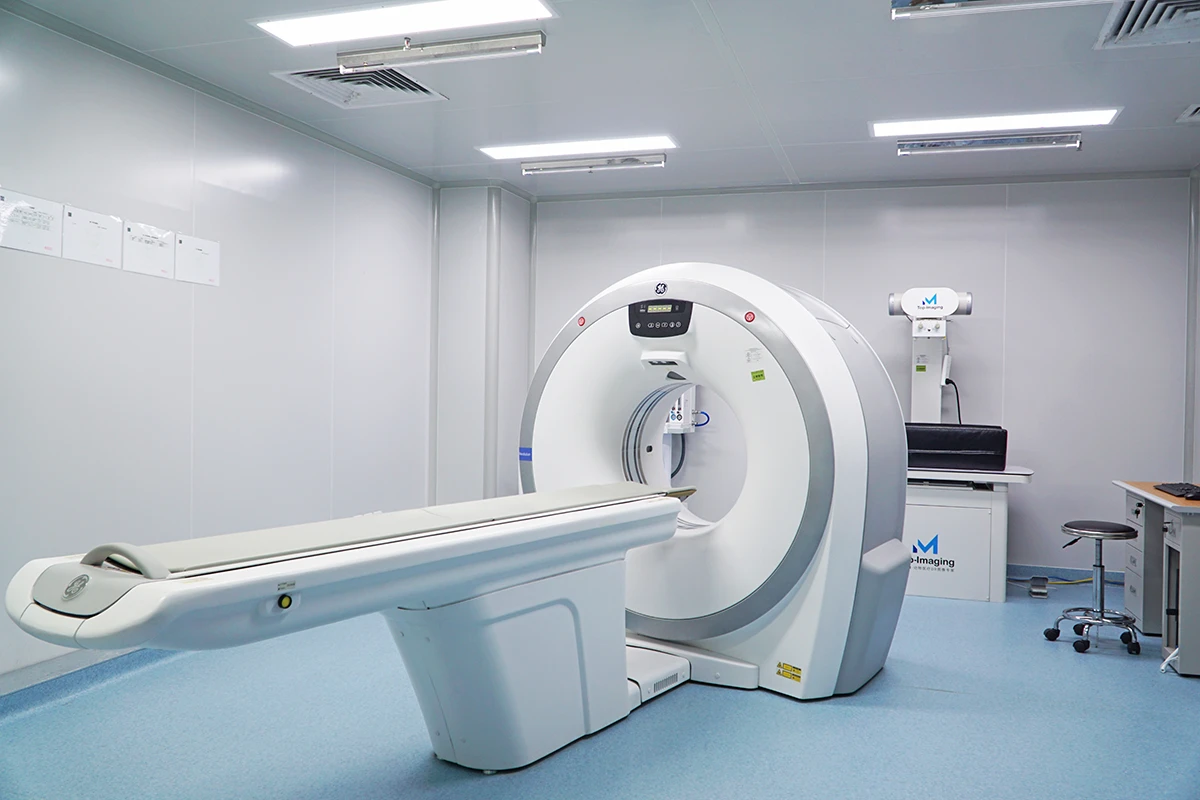
Our AAALAC-accredited facilities execute studies aligned with ISO 10993 (biocompatibility) and FDA/EMA/NMPA regulatory protocols. We bridge translational gaps through:
· Anatomically predictive models (ovine/caprine orthopedics, porcine cardiovascular systems)
· Surgical excellence under full IACUC oversight
· Human-correlated endpoints (histomorphometry, µCT, biomechanical testing)
GLP Large Animal Studies

GLP Large Animal Studies
AAALAC-accredited facilities | ISO 10993, FDA/EMA/NMPA aligned protocols | Species-specific physiologic relevance
HuaTeng Biotechnology delivers GLP-Compliant Large Animal Models for Comprehensive Medical Device Validation, providing scientifically rigorous preclinical data to de-risk regulatory pathways. As a trusted preclinical CRO, we specialize in orthopedic, cardiovascular, dental & active implant studies with species-specific physiologic relevance.