AAV8-1.3HBV Mouse Model


AAV8-1.3HBV Mouse Model
Huateng Bio offers AAV8-mediated HBV mouse models with 88% seroconversion rates and sustained viremia. Validated for antiviral drug testing and fibrosis research. Download virology and histopathology protocols.
Model Description
Hepatitis B virus (HBV) exhibits strict species specificity, limiting preclinical studies to non-human primates like chimpanzees, which face ethical and logistical constraints. Our AAV8-1.3HBV mouse model overcomes these challenges by delivering the full HBV genome via recombinant adeno-associated virus (rAAV8), enabling:
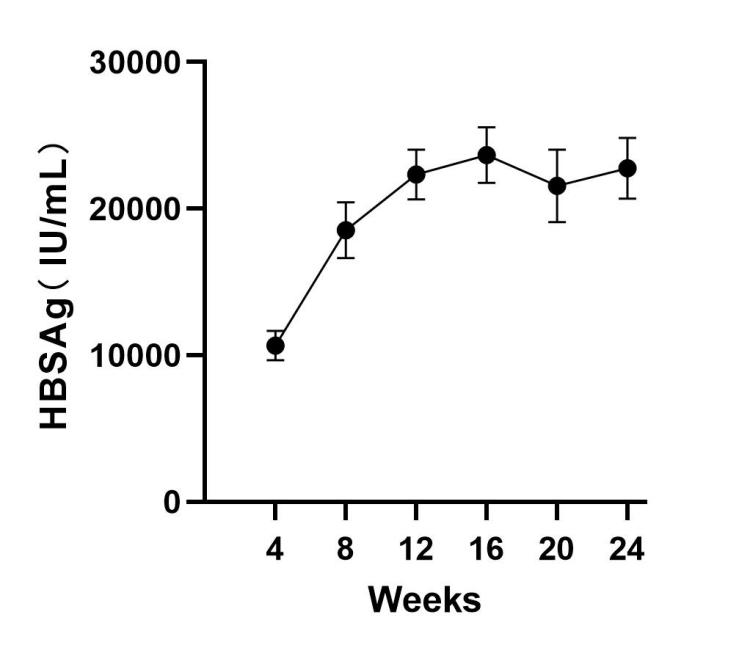
- High seroconversion rates: 88% HBsAg positivity within 4 weeks
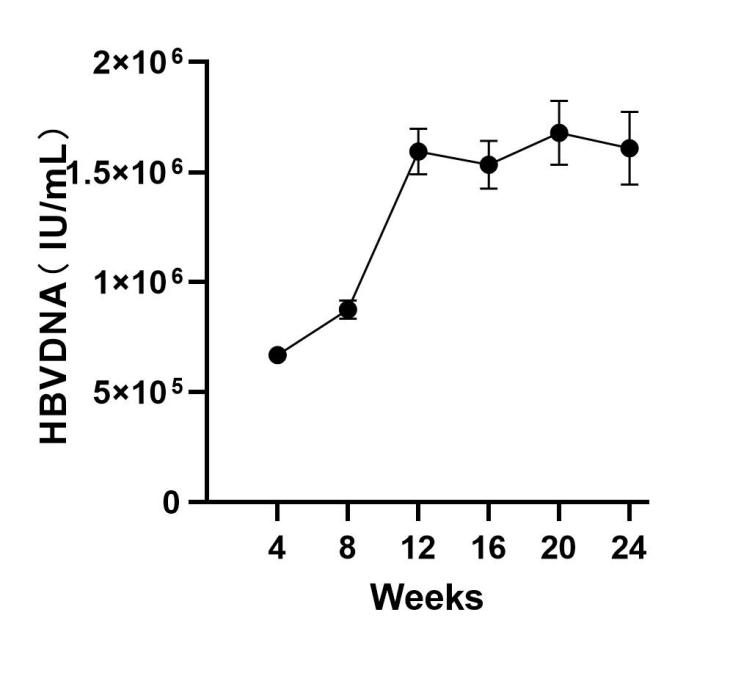
- Sustained viremia: Detectable HBsAg/HBeAg for ≥24 weeks
- Humanized pathology: Liver inflammation and fibrosis progression
Key Advantages vs Traditional Models:
✓ Cost-effective scalability vs primate models
✓ Controlled viral replication (1.3x HBV genome coverage)
✓ Patent-protected transgenic lines for comparative studies
Applications
• Antiviral drug screening (NAs, siRNA, capsid inhibitors)
• HBV vaccine efficacy testing
• Viral persistence mechanism studies
• Immune modulator development (TLR agonists, checkpoint inhibitors)
Modeling Protocol —— rAAV8-1.3HBV Tail Vein Injection
1. Vector Preparation:
- rAAV8-1.3HBV construct (1.3x overlength HBV genome, genotype D)
- Viral titer: 1×10<sup>12</sup> vg/mL
2. Procedure:
- Inject 100 μL vector via tail vein (1×10<sup>11</sup> vg/mouse)
- Monitor HBsAg seroconversion weekly (ELISA)
3. Modeling Timeline:
- Week 4: Peak HBsAg expression (≥500 IU/mL)
- Week 12-24: Chronic phase with liver fibrosis (METAVIR ≥F2)
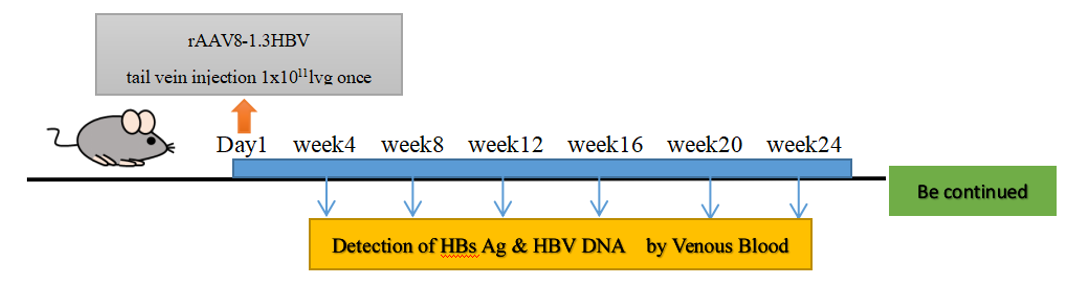
Fig.1 Construction of rAAV-1.3 HBV model
Validation & Testing
Category
Parameters
Virological Analysis
• Serum HBsAg/HBeAg ELISA ∙ HBV DNA qPCR (log<sub>10</sub> IU/mL)
Liver Function
ALT/AST levels ∙ Albumin/globulin ratio
Histopathology
H&E staining ∙ Sirius Red collagen quantification ∙ HBsAg IHC
Immune Profiling
Flow cytometry: CD8+ T cells ∙ PD-1/TIM-3 exhaustion markers
Molecular Analysis
cccDNA quantification (Southern blot) ∙ HBV RNA RT-PCR
Success Criteria
✓ Seroconversion: HBsAg >5 IU/mL by Week 4
✓ Viral persistence: HBV DNA >10<sup>4</sup> IU/mL at Week 12
✓ Fibrosis progression: Collagen area ≥5% (Sirius Red)
Technical Advantages
Feature
Our Model
Traditional Models
Infection Rate
88% ± 5%
≤50% (hydrodynamic injection)
Chronicity
24+ weeks
8-12 weeks
Clinical Relevance
Genotype D (global dominant)
Limited genotype coverage
Data