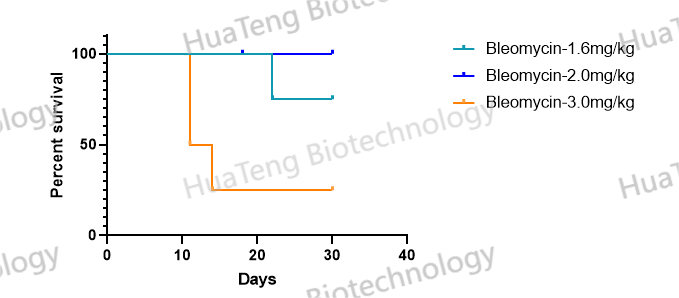
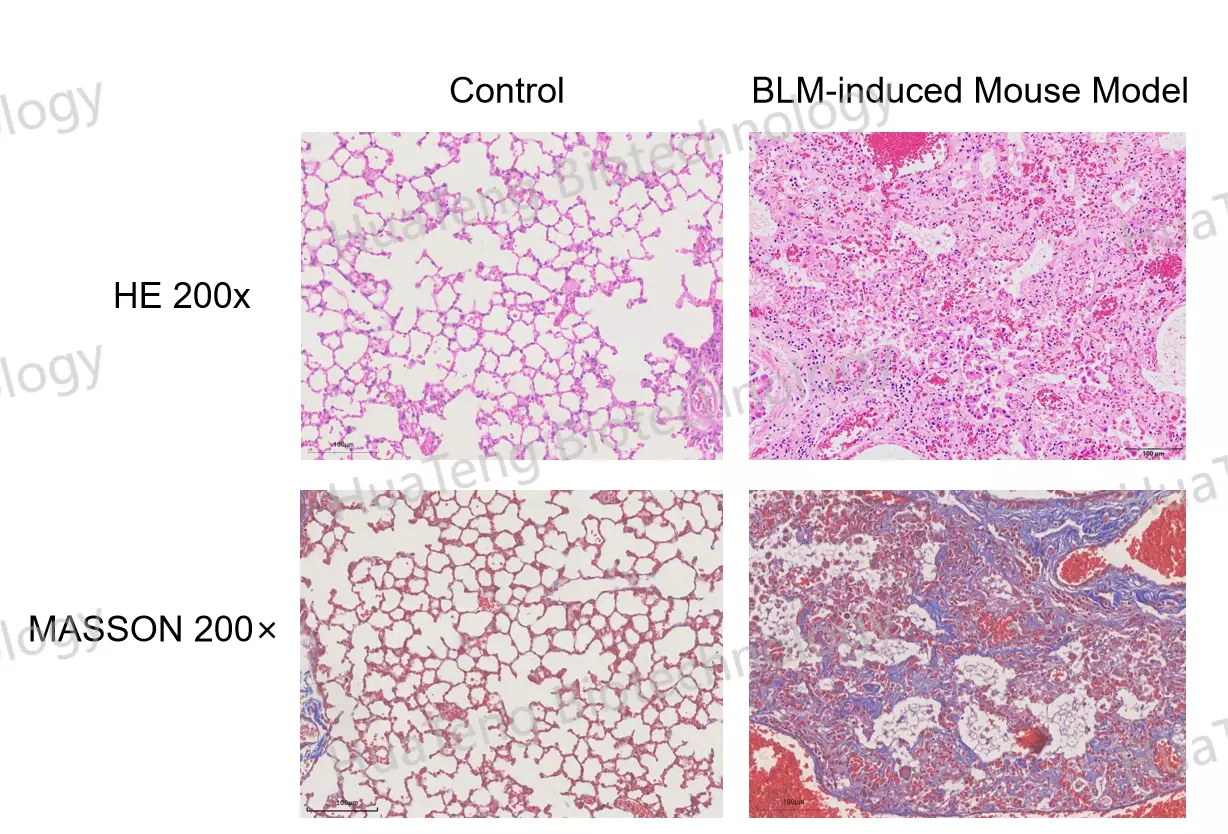
Bleomycin-Induced Pulmonary Fibrosis Mouse Model


Bleomycin-Induced Pulmonary Fibrosis Mouse Model
HuaTeng Biotechnology's BLM-induced pulmonary fibrosis models replicate human IPF pathology. Features dose-optimized bleomycin administration, micro-CT fibrosis quantification & cytokine profiling.
Model Name
BLM-induced pulmonary fibrosis
Animal Strains
C57BL/6
Our bleomycin (BLM)-induced pulmonary fibrosis models employ clinically-relevant dosing regimens (1.5-2U/kg via oropharyngeal aspiration) to achieve progressive fibrogenesis. Certified by Ashcroft scoring and hydroxyproline assays, these models enable robust evaluation of anti-fibrotic therapeutics, with pathological features including:
-
Alveolar architecture destruction
-
Masson's trichrome-positive collagen deposition
-
Sustained TGF-β1 upregulation
Technical Highlights
Parameter Specification Strains C57BL/6, BALB/c (SPF grade) Induction Method Orotracheal BLM (Single/Multiple dosing) Model Duration 28 days Key Validation ① Micro-CT fibrotic volume ② Hydroxyproline ③ Ashcroft score ④ α-SMA IHC Drug Testing Oral/Nebulized compound administration support