In the important journey of biopharmaceutical innovation, a group of quiet protectors stands watch. They guard the key step before human clinical trials: Non-Human Primates (NHPs). Primarily cynomolgus and rhesus macaques, these advanced laboratory animals serve as the cornerstone of predictive scientific research in drug development.
Using these methods requires a big investment. The history of drug companies reveals an important fact. Skipping strict animal testing in early research often results in failures. These failures can cost much more during clinical stages. This makes NHP studies not an optional expense, but a vital investment in safety and efficacy.
A Historical Imperative: The Cost of Ignoring Species Fidelity
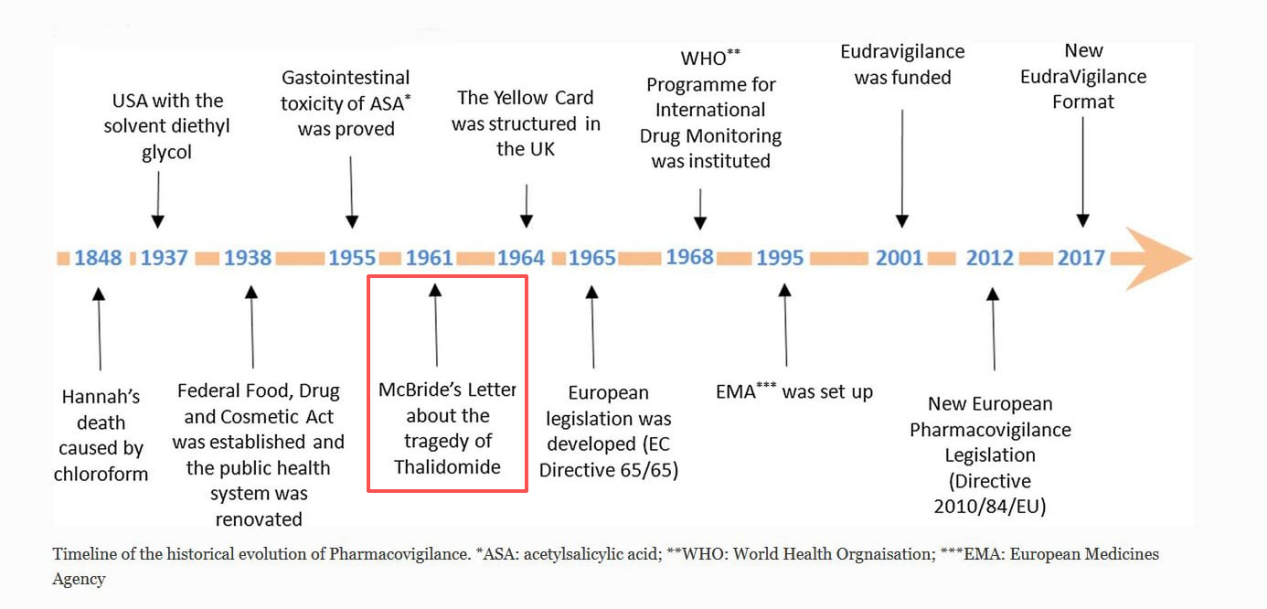
The thalidomide tragedy of the 1950s remains a painful lesson in medical history, fundamentally shaping modern toxicology studies and regulatory oversight. This event underscored a fatal flaw in relying solely on rodent models for safety evaluation, revealing profound species differences in drug metabolism and toxicity.
History and Evolution of Pharmacovigilance
It cemented the non-negotiable principle that for high-risk modalities, biological models closest to human physiology are essential. This historical imperative continues to drive the basic research and applied animal studies that underpin all clinic researches, ensuring that clinical study early stage trials proceed on a foundation of reliable safety data, aligned with Food and Drug Administration (FDA) expectations for proving a therapy is safe and effective.
The Irreplaceable Value of NHP Models in Modern Drug Development
The role of NHPs has become more distinct and critical with the advent of complex therapies, supporting a wide range of medical researches across three core areas where their physiological similarity is paramount.
1. The Ultimate Warning System for Complex Toxicity
Many drug-induced toxicities involve biological mechanisms unique to higher primates. NHP models are unparalleled in detecting these risks.
- Neurotoxicity: Advanced imaging and biomarker analysis in NHPs can non-invasively identify brain inflammation or edema caused by novel immunotherapies—risks other models miss.
- Metabolic & Off-Target Toxicity: Studies in NHPs can detect subtle interference with energy metabolism or other organ systems, providing a sensitive readout for safety evaluation far beyond rodent capabilities.
- Reproductive & Developmental Toxicity: For biologics and other long-acting therapies, NHP models provide essential data on placental transfer and fetal development critical for regulatory approval.
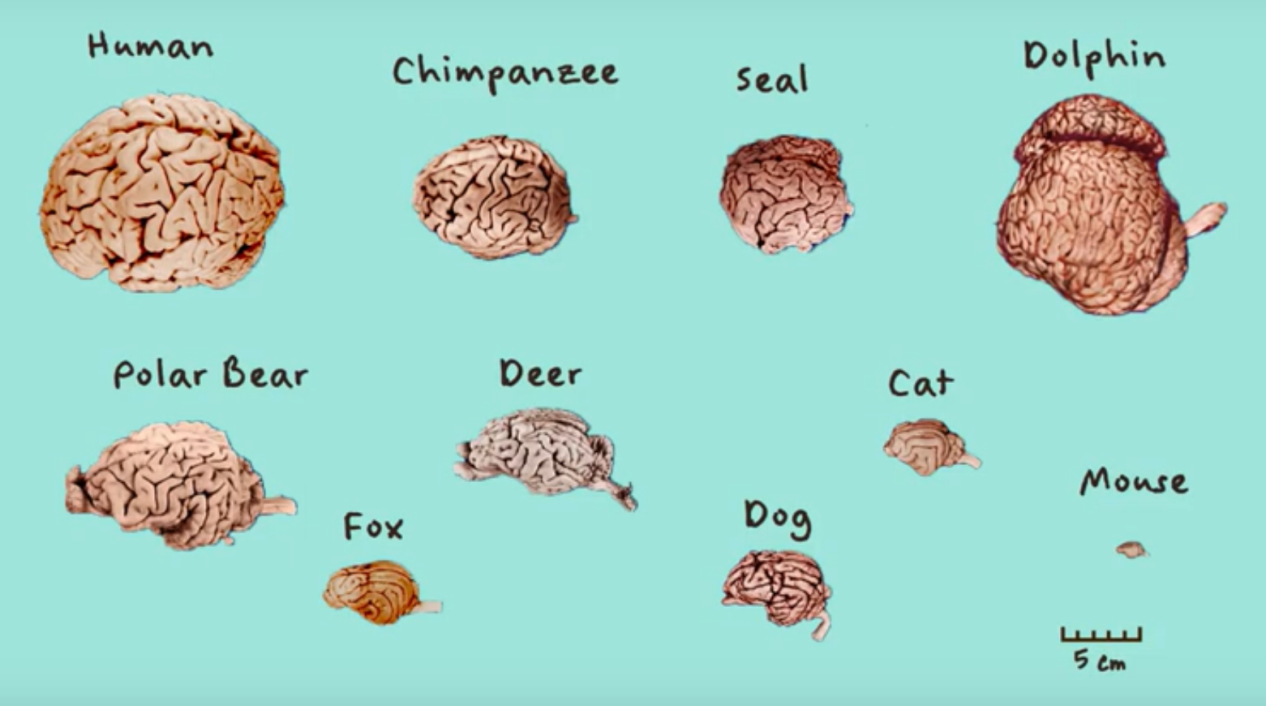
Comparison of the Human Brain with the Brains of Different Animals
2. The Essential Proxy for Biologics, Vaccines, and Gene Therapies
This area represents the highest demand and lowest replaceability for NHP animal research.
- Target Homology: Key drug targets in immunology and oncology often have identical structures in humans and NHPs, making them the only viable model for assessing sophisticated biologics gene therapy candidates and vaccine development platforms.
- Predicting Clinical Immune Response: The immune response to viral vectors, cell therapies, and vaccines in NHPs is the most reliable predictor of human clinical behavior. NHP "challenge studies" for infectious disease candidates offer decisive evidence on efficacy and pathology.
- Evaluating Long-Term Biological Effects: Understanding a therapy's interaction with the long-term immune systems and potential for immunogenicity is most accurately assessed in NHPs.
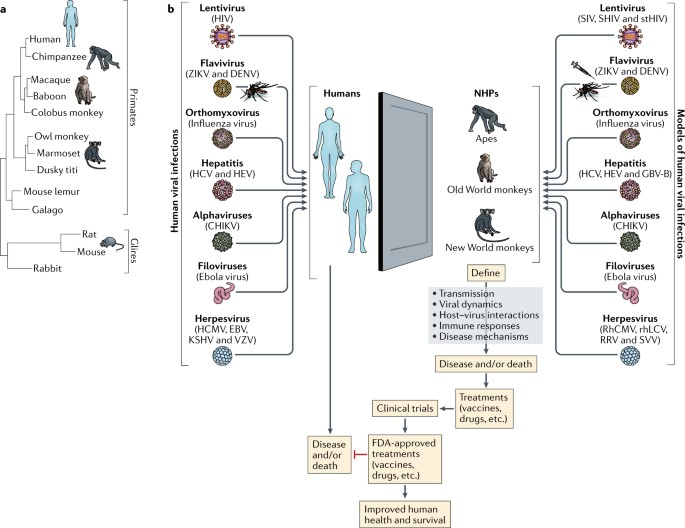
Using Non-Human Primate Models to Study Human Viral Infections
(https://doi.org/10.1038/s41577-018-0005-7)
3. The Long-Term Safety Simulator
For chronic disease treatments, NHP studies lasting several months offer an irreplaceable view of delayed effects.
- Bioaccumulation: NHP studies can track the slow accumulation of advanced modalities like ADCs in organs, revealing sub-cellular changes.
- Chronic Immunogenicity: The risk of developing neutralizing anti-drug antibodies and related complications is best evaluated in the primate immune system.
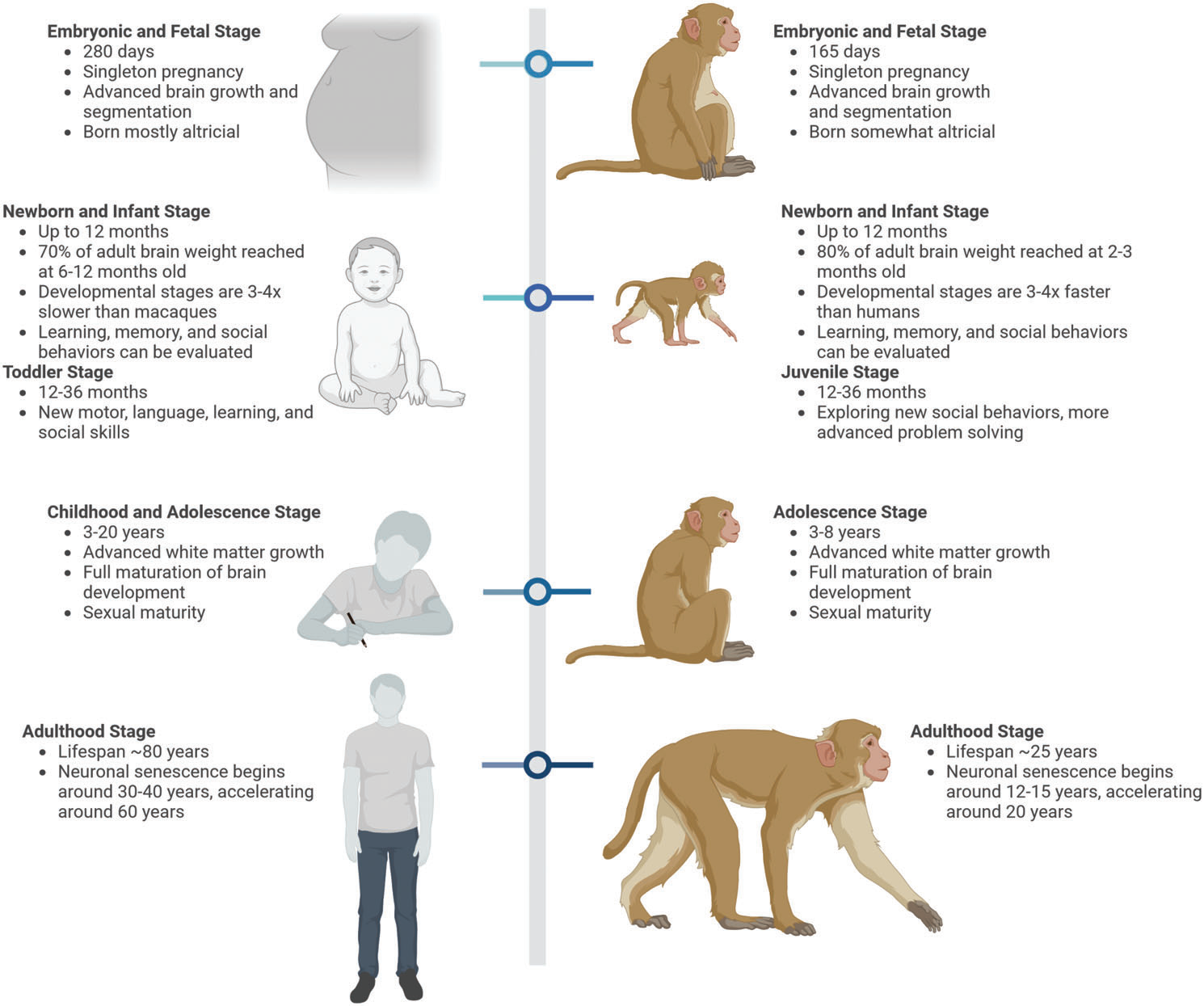
Humans and monkeys exhibit a highly similar life course in terms of growth and developmental processes
(https://doi.org/10.1002/cpz1.698)
The Strategic Economics: Prudent Investment vs. Catastrophic Risk
The substantial cost of an NHP study is strategically justified. It acts as a risk-hedging "insurance policy." A direct cost-benefit analysis is revealing: the financial and temporal cost of a Phase I clinical failure—often exceeding 10x the NHP study cost—coupled with a lost 18-24 month market window, represents a far greater burden.
For pharmaceutical companies, a strategic, deep mechanistic NHP study (costing 120-150% of a baseline compliance study) provides proactive risk identification and a clear mitigation plan, offering vastly higher assurance than a basic compliance report that may carry hidden hazards into human trials.
The Future: Ethical Refinement and Complementary Innovation
The field is committed to the ethical evolution of animal experimentation. This pursuit follows two paths:
- Refinement of NHP Use: Adhering to the highest welfare standards, techniques like micro-sampling and advanced imaging maximize data yield per animal.
- Development of Alternative Technologies: Computer models, organ-on-a-chip systems, and sophisticated in vitro platforms are rapidly advancing. These tools are invaluable for early screening and basic research, reducing animal use where possible.
However, for the integrated, systemic safety evaluation of the most complex drugs—especially those involving intricate immune interactions or multi-organ effects—the nonhuman primate model remains the scientific and regulatory gold standard. It is the critical system that stands between promising scientific research and the successful, safe and effective application of new medicines in clinic researches.
The HuaTeng Biotechnology Approach – Plan Your Preclinical Research with Us
At HuaTeng Biotechnology, we understand that robust NHP data is the ballast stone for your R&D voyage. We provide the scientific rigor, ethical commitment, and operational excellence required to make this critical investment truly effective.
Our world-class platform, adhering to international standards (AAALAC, OECD GLP), is designed to deliver the definitive preclinical insights you need to de-risk your most innovative programs and anchor your development strategy in safety and predictability.
[Contact HuaTeng Biotechnology] today and let us help you turn your research into success.